(Histologic basis for colposcopic findings)
The Normal Epithelium
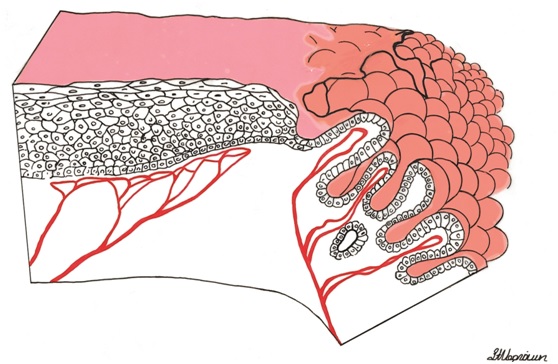
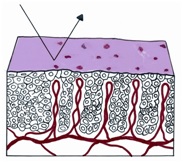
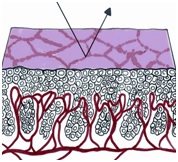
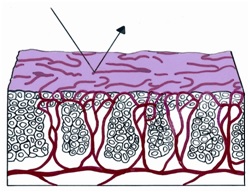
If a strip of normal stratified squamous epithelium is interposed between the strong light of the colposcope and the observing eye, the image formed is the result of the light transversing the superficial glycogenated cells and the basal layers of the squamous mucosa to reach the underlying lamina propria. Thus the reflected image will be influenced by the presence of intravascular red blood cells and will be in the red color range. The thicker the epithelium, the paler the red hue. The thinner the epithelium, the redder the image; normally, no blood vessels extend into the epithelium except for those in the stromal papillae confined to the basal zone of the epithelium.
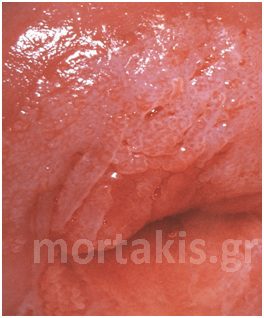
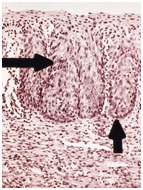
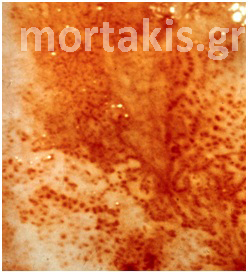
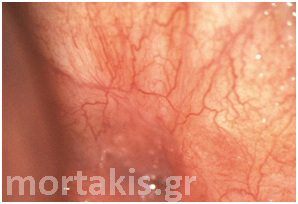
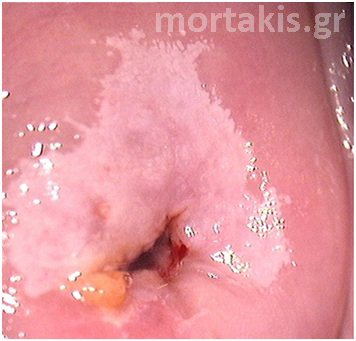
In each of the grapelike structures of the columnar epithelium, there is a bundle of capillaries that are separated from the observer by just one layer of columnar cells. This explains why the columnar epithelium looks intensely red to the naked eye (figures 1,2).
Figure 1 Histologic basis for colposcopic view of squamous and columnar epithelium of the cervix.
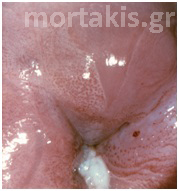
Figure 2 Colposcopic view: columnar cervical epithelium in the center looking intensely red and mature squamous epithelium at the periphery. Note thin cervical vessels above a Nabothian cyst at the lower left of the photograph.
Colposcopic appearance of Metaplastic and Atypical epithelium
The colposcopic morphology of the atypical epithelium harboring CIN is dependent on a number of factors. These include:
- Thickness of the epithelium – a result of the number of cells and their maturation.
- Variations in blood vessel patterns.
- Alterations in surface contour and any associated changes in the covering epithelium (keratinization).
Acetowhite epithelium
When cervical epithelium appears grossly normal but it turns white after the application of acetic acid solution (3-5%), white areas are called acetowhite epithelium.
How acetic acid works as a contrast agent is unclear. It can modify cellular proteins, including cytokeratins and nuclear proteins. Confocal microscopy before and after the application of acetic acid has demonstrated an increased nuclear signal, which implies increased light scattering by nuclear material. Lastly it is believed (but not yet proved) that acetic acid dehydrates the cell, which is left with organelles, cytoskeleton filaments, and nuclear proteins. In this way the tissue appears more “dense”. Abnormal epithelial cells contain an increased amount of protein, and application of dilute acetic acid results in overlapping of the enlarged nuclei. Light is not able to pass through the epithelium and is reflected back at the colposcopist, appearing white.
There can be varying degrees of acetowhiteness, depending on epithelial thickness and degree of nuclear enlargement and density.
The change is transient and reversible. It can be renewed by the reapplication of acetic acid. The intensity of the whiteness, its speed of appearance, its duration, and the rapidity of its disappearance are all related to the number of immature, abnormal or neoplastic cells. The greater the number of such cells, the more intense the whiteness, the faster the change will develop, and the longer its duration.
The acetowhite changes are the most important of all the colposcopic features because they are associated with all grades of CIN.
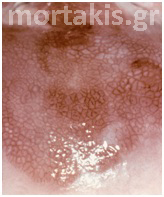
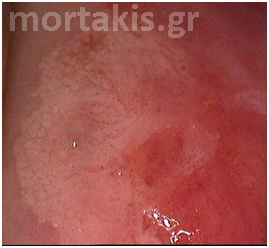
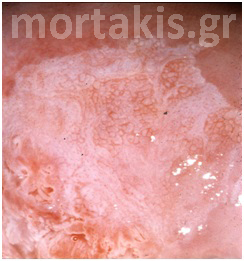
Colposcopists evaluate the color and density of the acetowhite reaction to assess the severity of the lesion. Acetowhite epithelium varies from a faint or a bright white (immature metaplasia and low-grade changes) to a dense gray white (high-grade lesions). Color is somewhat subjective, and therefore may be hard to classify. Variations of white can be even more difficult to describe. Glare also may influence the determination of color. The dilemma of color description is further affected by the varied illumination sources for colposcopes, which emit slightly different wavelengths or shades of white light (figures 3-10).
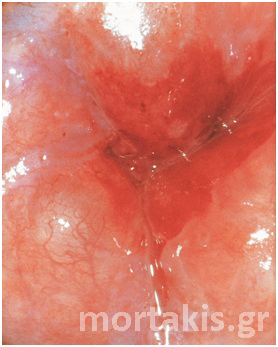
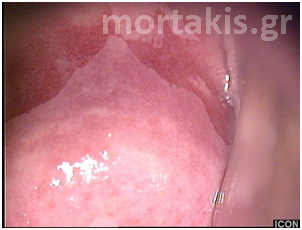
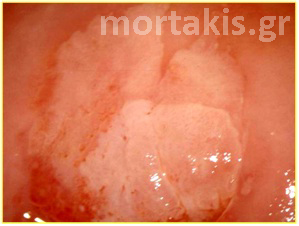
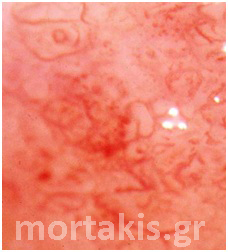
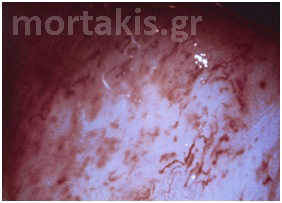
Figure 3 Acetowhite epithelium of immature squamous metaplasia ( light reflections at the upper part of the photo).
Figure 4 Translucent acetowhiteness of metaplastic epithelium
Figure 5 Translucent acetowhite metaplastic epithelium (vaginal vault – congenital transformation zone).
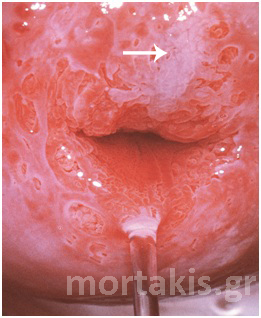
Figure 6 Striking acetowhite epithelium (snow like appearance) of a LSIL.
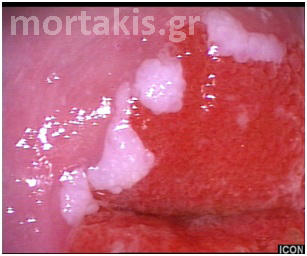
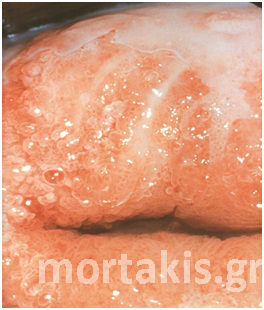
Figure 7 Cervical condylomatous lesions.
Figure 8 LSIL. Condylomatous lesions of the cervix. Bright acetowhite epithelium, micropapillary surface of the lesions.
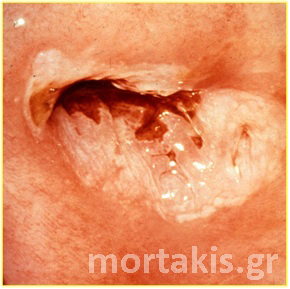
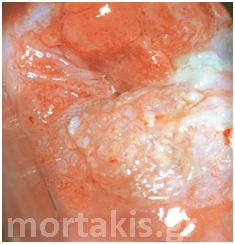
Figure 9 Acetowhiteness of a HSIL. Opaque, grey-white of a CIN3 lesion.
Figure 10 Acetowhite epithelium of a HSIL. Opaque acetowhiteness. The lesion is clear demarcated from the surrounding normal squamous epithelium)
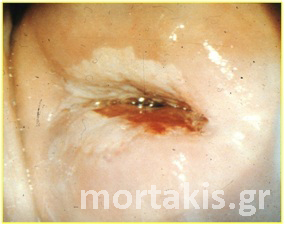
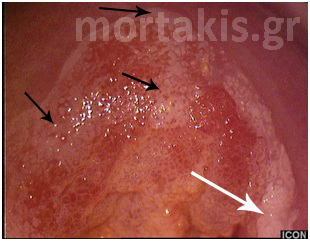
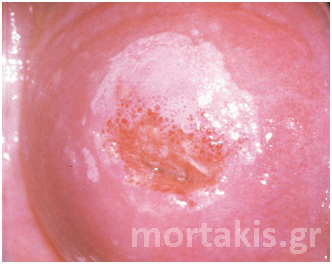
As you can see at figure 11, it is possible to have varying degrees of acetowhiteness within the same lesion, with peripheral faint acetowhite change accompanied by a central dense acetowhite reaction. This finding is known as an internal margin, and it may be associated with significant high-grade lesions.
Figure 11. “Lesion within a lesion” (dense white of a CIN3 lesion in the center near the cervical os, faint acetowhiteness in the periphery – CIN1)
Not all epithelium that turns acetowhite is abnormal. Any cell with an enlarged nucleus, such as metaplastic cells or cells traumatized by infection or friction (regenerative changes) may exhibit varying degrees of acetowhiteness. The intensity of acetowhiteness does not always correlate with the severity of the lesion: Condyloma may have a striking white appearance of very rapid onset whereas tissue harboring CIN3 or microinvasive changes may appear a thick white or a grayish color respectively. Thus it may be impossible to differentiate between benign and neoplastic findings, in any case, and biopsy is the only solution, whenever the colposcopist cannot be sure.
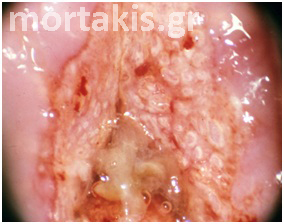
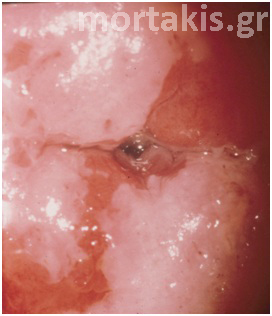
It is important to determine whether the acetowhite reaction is present on the squamous or columnar epithelium. If the columnar epithelium exhibits an acetowhite reaction, it may represent normal metaplastic epithelium (figures 12, 13), atypical metaplastic epithelium (figure 14) or a glandular epithelial abnormality (figure 15). If intraepithelial neoplasia is present at the os of a glandular crypt, it may appear as a white-cuffed gland opening. These cuffed gland openings should be easily distinguished from the faint rim of metaplastic epithelium surrounding normal gland openings.
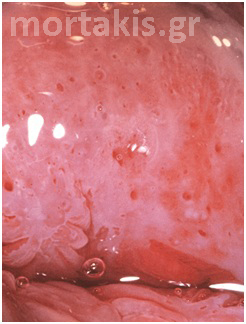
Figure 12 Normal metaplasia at an early stage.
Figure 13 Normal metaplasia at a late stage.
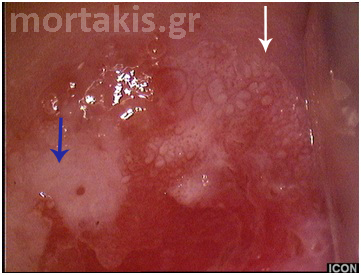
Figure 14 Metaplastic epithelium (small arrows) covering areas of columnar epithelium (immature squamous metaplasia). A CIN2 lesion (big arrow) in an area of metaplastic epithelium. Note the translucent acetowhiteness of metaplastic epithelium and the opaque acetowhiteness of the CIN2 lesion.
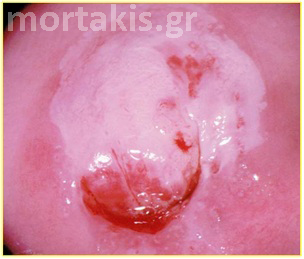
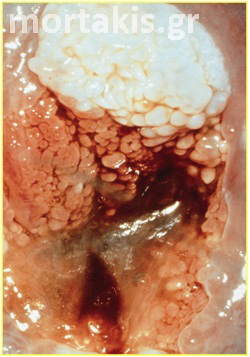
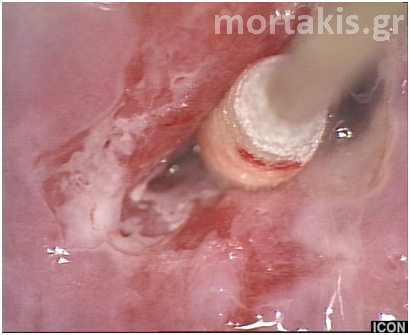
Figure 15 Cervical adenocarcinoma. The picture is quite different from normal metaplasia.
Punctation and Mosaic Patterns
If we combine a block with squamous cells with a change in vascularity in which the blood vessels, rather than being confined to lamina propria, extend to the surface of the epithelium, a distinctive pattern called punctation will be identified. After the application of acetic acid, the tips of the vessels will be seen colposcopically as red dots perforating the acetowhite epithelium (figure 16 a, b, c).
Figure 16 (a, b, c) Histologic basis and colposcopic picture of punctation.
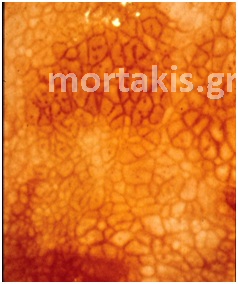
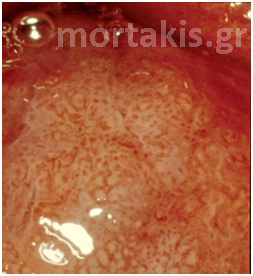
If the vasculature does not reach the surface, however, but rather extends only partially through the epithelium, it forms a basketlike network around the blocks of immature cells and a distinctive colposcopic image called a mosaic structure ensues. After the application of acetic acid, the top of the basketlike arrangement of vessels that surrounds the abnormal blocks of cells is identified as a red line. The appearance is reminiscent of tiles on a floor and, thus, the term “mosaic” is used to describe the image (figure 17 a, b, c).
Figure 17 (a, b, c) Histologic basis and colposcopic picture of mosaic
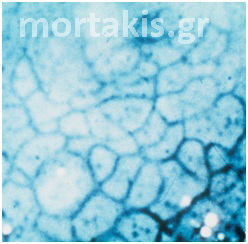
Punctation and mosaic can be seen in both normal and abnormal cervical epithelium. Abnormal vessels can be visualized with a red-free (green-filtered) light.
Examples of non neoplastic epithelium exhibiting punctation, mosaic or both include inflammatory conditions such as trichomoniasis, gonorrhea or chlamydial infection or very active immature squamous metaplasia. If the punctation or mosaic is not located in a field of acetowhite epithelium, it is unlikely to be associated with CIN.
Figure 18 shows the beginning of atypical squamous metaplasia. The vascular structures are not connected, and metaplastic epithelium completely fills the folds and clefts of the columnar epithelium. Colposcopically, we see reddish fields separated by whitish borders; this colposcopic finding is called reverse mosaic.
Figure 18 Reddish fields separated by whitish borders (reverse mosaic pattern). LSIL in an area of immature metaplasia. Note satellite LSIL lesions on the mature squamous epithelium.
The punctation or mosaic pattern is described as fine or coarse. If the vessels are fine in caliber, regular, and located close together (small intercapillary distance), it is more likely that the patterns represent a benign process (metaplasia) or low-grade CIN (figures 19, 20).
If the intercapillary distance of the vessels is increased and they are coarser in appearance, the grade of the lesion is usually more severe, and it is unlikely that a benign process is present. Generally the distance between capillary loops (intercapillary distance) increases as the amount of cell proliferation increases. As such, the greater the grade of CIN, the greater the distance between the capillary loops. Coarse punctation, seen in HSIL, is characterized by large irregularly sized dots that may appear above the epithelial surface. The intercapillary distance is increased and the spacing is uneven (figures 21-23).
Continued production of angiogenic factors in the presence of persistent cell production results in further vascular growth. The capillary loops begin to arborize and coalesce beneath the surface. Mosaicism is a natural progression from punctation, and it is common to see evidence of punctuate dots adjacent to or within an area of mosaicism. The tiles of mosaic show irregular shapes and varying sizes (figures 24, 25).
Figure 19 Fine punctation and mosaic of immature metaplastic epithelium (fine caliber of the vessels, small intercapillary distance)
Figure 20 Fine punctation and mosaic of a LSIL
Figure 21 Coarse punctation of a CIN 3 lesion. Large diameter of the vessels.
Figure 22 Coarse mosaic of a CIN 3 lesion. Note coarse punctation in the tiles of mosaic.
Figure 23 Coarse punctation and reverse mosaic of a HSIL
Figure 24 Coarse mosaic (green filter). Tiles of variable sizes with punctation prominent in the center of some tiles. Asymmetry (Carcinoma in situ).
Figure 25 Coarse punctation (green filter). Note the large caliber of the vessels, variable diameters, and asymmetry. Note also, appearance of small atypical surface vessels (final diagnosis: Microinvasion).
It should be emphasized that many preinvasive lesions lack abnormal vessels and are identified only by the presence of acetowhite epithelium (figures 9, 10, 11).
Atypical Blood Vessels
The neoplastic epithelium has high metabolic needs, but its own growth compresses the vessels that supply it. Tumors cannot grow beyond a few hundred thousand cells unless new capillaries develop. This process of new vessel formation by the tumor is called angiogenesis. These vessels do not display the normal arborizing vessel patterns.
As normal vessels divide, their caliber progressively decreases in size. Figures 26-29 are colposcopic photographs of normal cervical vessels.
Atypical vessels can paradoxically increase in size as they separate. This is because, to keep up with continued tumor expansion, the newly established vessels lose their consistent branching patterns and are now arranged haphazardly, as you can see at figure 30 (colposcopic picture of a squamous cell cancer of the cervix).
Atypical vessels do not have a uniform appearance. These nonarborizing vessels are recognized colposcopically as having “corkscrew”, “comma”, “noodlelike”, “rootlike” or “hairpin” patterns (figures 31-34).
The term “atypical vessels” is considered pathognomonic of colposcopic impression of carcinoma and must be used cautiously. If unusual angiogenic patterns are seen that do not necessarily imply malignancy, then other descriptors should be used, in a colposcopy report.
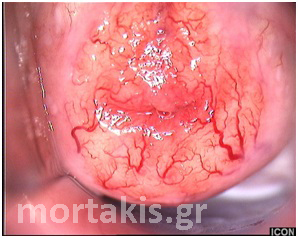
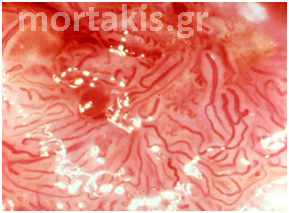
Figure 26 Normally arborizing cervical vessels. As normal vessels divide, their caliber progressively decreases in size.
Figure 27 Normally arborizing vessel patterns of the cervical epithelium over a Nabothian cyst.
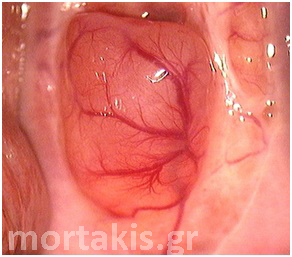
Figure 28 Normal cervical vessels over multiple Nabothian cysts.
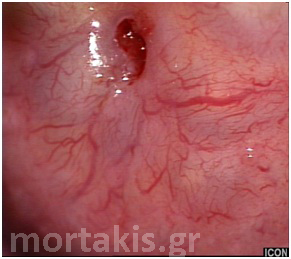
Figure 29 Normally branching cervical vessels (atrophic epithelium).
Figure 30 Atypical vessels of squamous invasive cervical cancer (“corkscrew”, “noodlelike”, “rootlike” and “hairpin” patterns).
Figure 31 Histologic basis for atypical cervical vessels
Figure 32 Atypical vessels (microinvasion). Irregular arborization, “spaghetti” like appearance.
Figure 33 Atypical vessels of invasive cancer (“root like” and “commas” appearances)
Figure 34 Atypical vessels of invasive cancer (they paradoxically increase in size as they separate) with bizarre patterns.
Leukoplakia and Keratosis
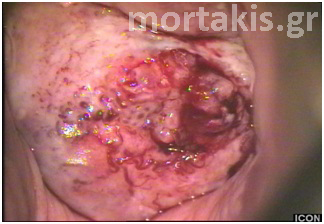
If the squamous epithelium has plaques of keratin on its surface, the light cannot traverse the epithelial cells and reach the blood of the vessels in the lamina propria. Thus, rather than being red, the visual image is a white plaque (figures 35, 36).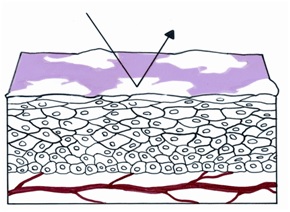
Figure 35 Histologic basis for leukoplakia
Figure 36 Colposcopic picture of a squamous invasive cervical cancer. The posterior lip of the cervix is covered with keratin.
Because leukoplakia (white plaque) is visible before the application of the acetic acid solution, it is differentiated from acetowhite epithelium that appears white only after the application of acetic acid.
Surface pattern and margins of the lesions
The surface pattern of the lesion may be smooth or irregular. With the exception of condylomatous lesions, surface irregularity is indicative of high-grade disease or invasion.
With increasing severity of the lesion, the edge definition (margin of the lesion) becomes sharper.
Condylomatous lesions may vary in surface contour, from flat lesions with fine punctation, to slightly raised areas with asperities, to florid, exophytic condylomata acuminata (figures 7, 8, 37).
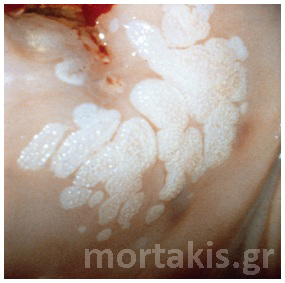
Figure 37 Exophytic condyloma acuminatum of the cervix.
In non-condyloma-like LSILs, the surface contours are usually flat. The margins may be indistinct, with the acetowhite changes noted at the squamocolumnar junction fading into the background color of the mature squamous epithelium (figures 38, 39). The margins may also appear irregular, as opposed to the sharp, straight margins of HSILs (figure 40).
With high-grade CIN, a decreased number of desmosomes is present, thus accounting for the finding of peeling edges and true erosions (figure 41). The epithelium is actually peeling off the underlying membrane, producing erosion or rolled lesion margin.
There may be more than one border apparent within the transformation zone. These latter “internal borders” may demarcate areas of high-grade CIN within a background of lower grade changes (figure 11).
Figure 38 Smooth surface and ill-defined margins are usually characteristics of metaplastic epithelium or LSIL
Figure 39 Smooth surfaces. On the right, metaplastic epithelium (translucent acetowhite) with ill defined margins, fine punctation and mosaic (white arrow). On the left, LSIL with mild acetowhiteness and better defined margins (blue arrow)
Figure 40 Well defined margins of a HSIL. Thick white epithelium, absence of vessels.
Figure 41 HSIL: Detachment of surface epithelium, due to the lack of desmosomes at the basement membrane. Thick white epithelium, lesion entering the canal.
Evaluation of the significant characteristics of the lesions
The above images of leukoplakia, acetowhite epithelium, punctation, mosaic pattern, and atypical blood vessels as well as topography of the lesion, size of the lesion, its margins and surface pattern are the basic descriptive vocabulary of the colposcopic method. Any process that increases keratin production, increases cellular division, increases vascular changes, and produces new blood vessels can cause any of the above images. Thus, metaplasia, infection, inflammation, regeneration, repairs and, most importantly, neoplasia can produce these changes. As we shall subsequently see, the changes of neoplasia can for the most part be distinguished from the less important changes causing these various colposcopic appearances. A skilled colposcopist should be able to distinguish minor from major changes by considering a wider range of the above characteristics in forming a diagnosis.
Colposcopic characteristics of the different stages of metaplasia
If the squamous mucosa consists of immature metaplastic cells, the co